Menu
Menu
Cancer research is continuously evolving, and new discoveries are helping us understand the complex mechanisms behind tumor development and progression. A recent study published in Nature Cell Biology highlights the role of a histone variant called macroH2A in melanoma, a type of skin cancer. This study offers detailed insights into how the absence of macroH2A influences the tumor microenvironment (TME) and immune response, potentially paving the way for new therapeutic strategies.
What is MacroH2A?
Histones are proteins that help organize DNA in cells, impacting how genes are expressed. MacroH2A is a unique histone variant with a special structure that can influence gene activity differently from other histones. Previous research has shown that macroH2A has tumor-suppressive functions in various cancers, including melanoma. However, its specific role in the TME was not well understood until now.
Study Overview
The study, led by Dan Filipescu and colleagues, used a mouse model that closely mimics human melanoma. These mice either had or lacked macroH2A proteins. Researchers found that mice without macroH2A (called double knockout or dKO mice) developed significantly larger tumors than those with normal macroH2A levels. This indicates that macroH2A plays a crucial role in controlling tumor growth.
Immune System and Tumor Growth
One key finding was how the immune system reacted in dKO mice. Normally, the immune system fights cancer, but in mice without macroH2A, tumors accumulated more immunosuppressive cells. These cells inhibit the immune system’s ability to attack the cancer effectively. As a result, there were fewer cytotoxic T cells, which are essential for killing cancer cells. This immunosuppressive environment allows tumors to grow more rapidly.
Chromatin Looping and Inflammatory Gene Expression
The researchers explored why this immune suppression occurs by examining the DNA structure in the tumor environment. They found that cancer-associated fibroblasts (CAFs) in dKO mice had increased chromatin looping. Chromatin looping is a structural arrangement that brings distant DNA regions close together, enhancing gene expression. In dKO mice, this increased looping led to higher expression of inflammatory genes such as CCL2, CXCL1, and IL-6. These genes attract immunosuppressive myeloid cells to the tumor, worsening the immune suppression and promoting tumor growth.
Advanced Techniques Reveal Detailed Insights
The study employed advanced techniques like single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics to analyze the tumor environment in detail. These techniques revealed that dKO mice had more dedifferentiated cells and more active CAFs. Dedifferentiated cells are less specialized and can contribute to tumor growth and resistance to therapy.
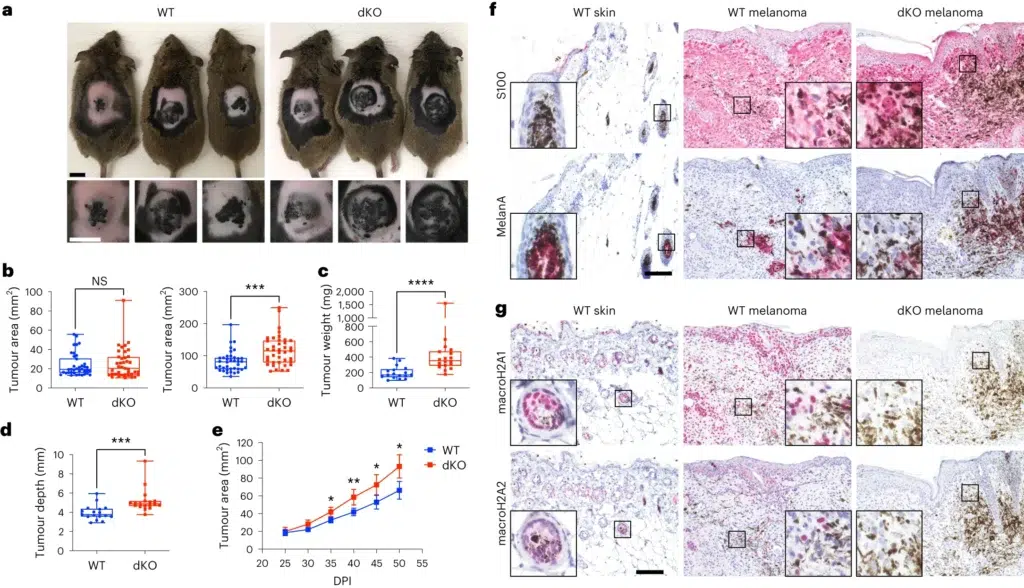
a, Macroscopic appearance of BRAFV600E/PTEN-deficient autochthonous melanomas in macroH2A WT mice and in dKO mice at 50 DPI. b, Comparison of tumour area across genotypes at the indicated time points. nWT = 38, ndKO = 39. P = 0.7962 at 25 DPI, P = 0.0002 at 50 DPI. c, Measured weight of resected tumours at the end point (50 DPI). nWT = 17, ndKO = 18. P < 0.0001. d, Average tumour depth calculated from the tumour area and volume at the end point (50 DPI). nWT = 17, ndKO = 18. For b–d, significance was determined using Mann–Whitney two-tailed test. Box plot whiskers represent the minimum to maximum range, the box plot limits the 25th to 75th percentiles, and the centre line the median. P = 0.0001. e, Tumour growth kinetics between 25 and 50 DPI. nWT = 22, ndKO = 22. Mean and 95% confidence interval error bars are shown. P values adjusted for multiple comparisons: *P < 0.05, **P < 0.01, Mann–Whitney two-tailed test. Exact P values are provided as numerical source data. f, Immunohistochemical characterization of normal dorsal skin and representative tumours in a. Antigens indicated are stained pink (Vector Red substrate). g, Immunohistochemical analysis as in f, but demonstrating macroH2A1 and macroH2A2 staining in normal skin and in WT and dKO melanoma. For f and g, insets are shown at additional ×4 magnification. Staining was repeated on nWT skin = 2, nWT melanoma = 7, ndKO melanoma = 6 mice with similar results. Scale bars, 100 μm (f,g) or 1 cm (a). NS, not significant.
Key Data and Findings
Implications for Cancer Therapy
The findings underscore the role of macroH2A in regulating the TME and maintaining an effective anti-tumor immune response. By controlling the pro-inflammatory properties of the tumor stroma, macroH2A helps suppress tumor growth. These insights suggest that targeting the pathways influenced by macroH2A could enhance cancer treatment. For example, therapies that inhibit the inflammatory signals or restore the immune system’s ability to attack the tumor could be developed.
This study provides a deeper understanding of macroH2A’s role in melanoma, showing how it helps control tumor growth and supports the immune system. These discoveries open new possibilities for developing treatments that enhance the body’s natural defenses against cancer. As research continues, these findings bring hope for more effective and targeted therapies for melanoma and potentially other cancers.
Read the full publication here
 100 Enterprise Way
100 Enterprise Way