Menu
Menu
Super-Enhancers and Their Role in Multiple Myeloma
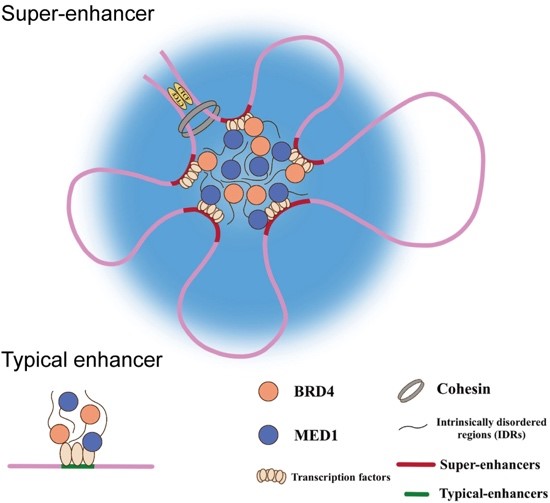
Enhancers are regulatory elements located throughout the genome that bind transcription factors and “enhance” transcription of target genes. However, not all enhancers are created equal. Large clusters of enhancer elements form what is termed a super-enhancer. As the term implies, super-enhancers exhibit heightened gene-activating potential compared to isolated enhancers and are often at the center of complex regulatory networks.
In multiple myeloma, super-enhancers are frequently hijacked, driving over-expression of oncogenes – genes that contribute to tumorgenesis. By integrating chromatin immunoprecipitation sequencing (ChIP-seq) with HiChIP, a modified Hi-C workflow, researchers have been able to map super-enhancers and their regulatory networks in both myeloma cell lines and patient-derived cells, revealing super-enhancer regulated genes. As a result of these efforts, PPP1R15B was identified as a key oncogene activated by a hijacked super-enhancer network in multiple myeloma.
The Role of PPP1R15B in Myeloma Cells
PPP1R15B encodes a regulatory subunit of the protein phosphatase 1 (PP1) complex involved in controlling protein synthesis. Central to this role, PP1 is critical for elF2a dephosphorylation and activation of the unfolded protein response (UPR), a pathway that reacts to the accumulation of misfolded proteins under stress conditions. When PPP1R15B is silenced, the UPR becomes overwhelmed leading to reduced protein production, activation of pro-apoptotic pathways, and cell death.
In multiple myeloma cells, maintaining elevated levels of immunoglobulin production rely on UPR pathways. Constitutively activated PPP1R15B is key to ensuring the UPR pathway does not become overwhelmed protecting the cell from programmed cell death.
Super-Enhancer Regulation of PPP1R15B
How are super-enhancers involved in constitutive activation of PPP1R15B? HiChIP data captures enhancer-promoter interactions orchestrated through long-range looping of chromatin in 3D space. The PPP1R15B promoter was observed to interact with a specific super-enhancer located upstream of the gene. As predicted, knocking out the super-enhancer using a CRISPR interference (CRISPRi) approach, resulted in reduced PPP1R15B expression. These findings underscore the crucial role played by super-enhancers in maintaining the oncogenic activity of PPP1R15B.
Therapeutic Potential of Targeting PPP1R15B
Given the essential role of PPP1R15B in sustaining protein homeostasis and cell survival in myeloma, it presents a promising therapeutic target. Pharmacological inhibition of PPP1R15B with the small molecule inhibitor Raphin1 has shown potent anti-myeloma effects. Raphin1 works by blocking PPP1R15B activity, thereby halting protein synthesis and triggering apoptosis in myeloma cells. Moreover, synergistic benefits are observed when combined with current standard of care treatments, such as the proteasome inhibitor bortezomib, leading to increased stress and programmed cell death.
Conclusion and Future Directions
By integrating the protein-directed interactome data captured by HiChIP, a modified Hi-C workflow, researchers were able to link a known oncogene, PPP1R15B, to its controlling super enhancer. This work provides a framework for detangling the complexities of oncogenic gene expression. Understanding the role of super-enhancers in driving oncogenic programs opens new avenues for targeted therapies, not only in multiple myeloma, but also in other cancer types. By exploiting the dependence of myeloma cells on PPP1R15B for managing protein synthesis under stress, this novel therapy promises to improve patient outcomes.
Full article: https://www.nature.com/articles/s41467-024-50910-z
 100 Enterprise Way
100 Enterprise Way